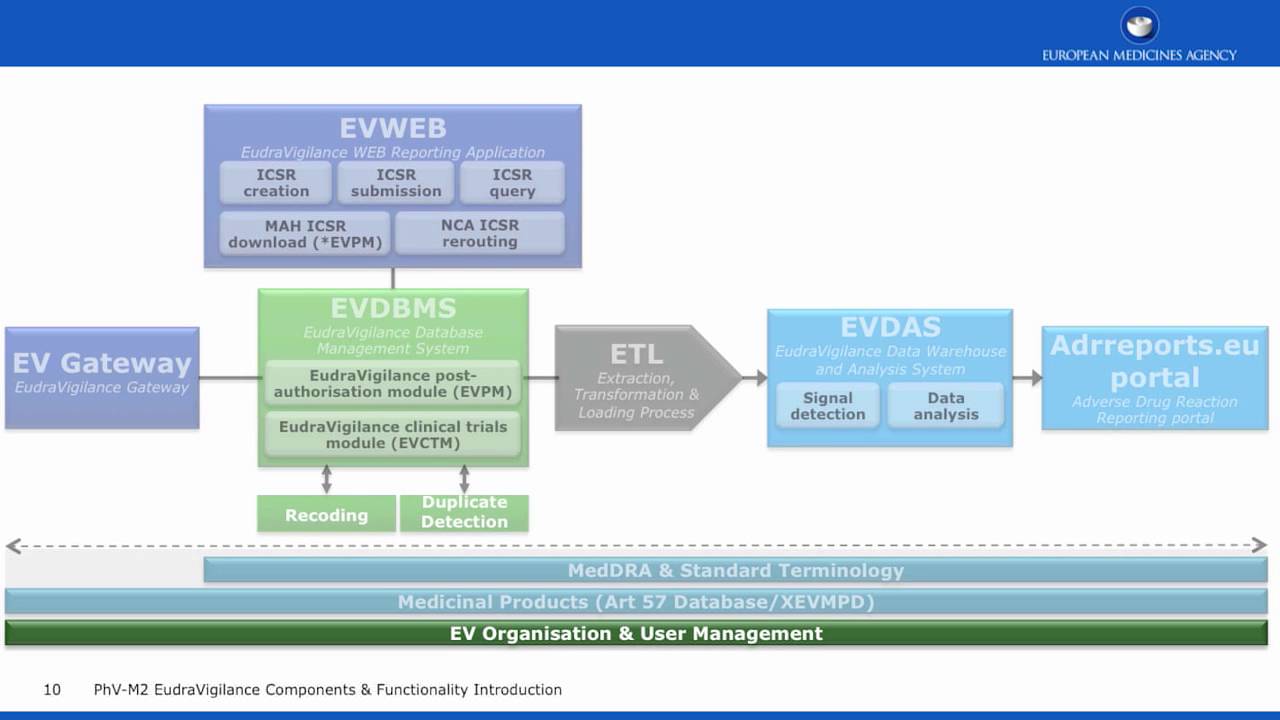
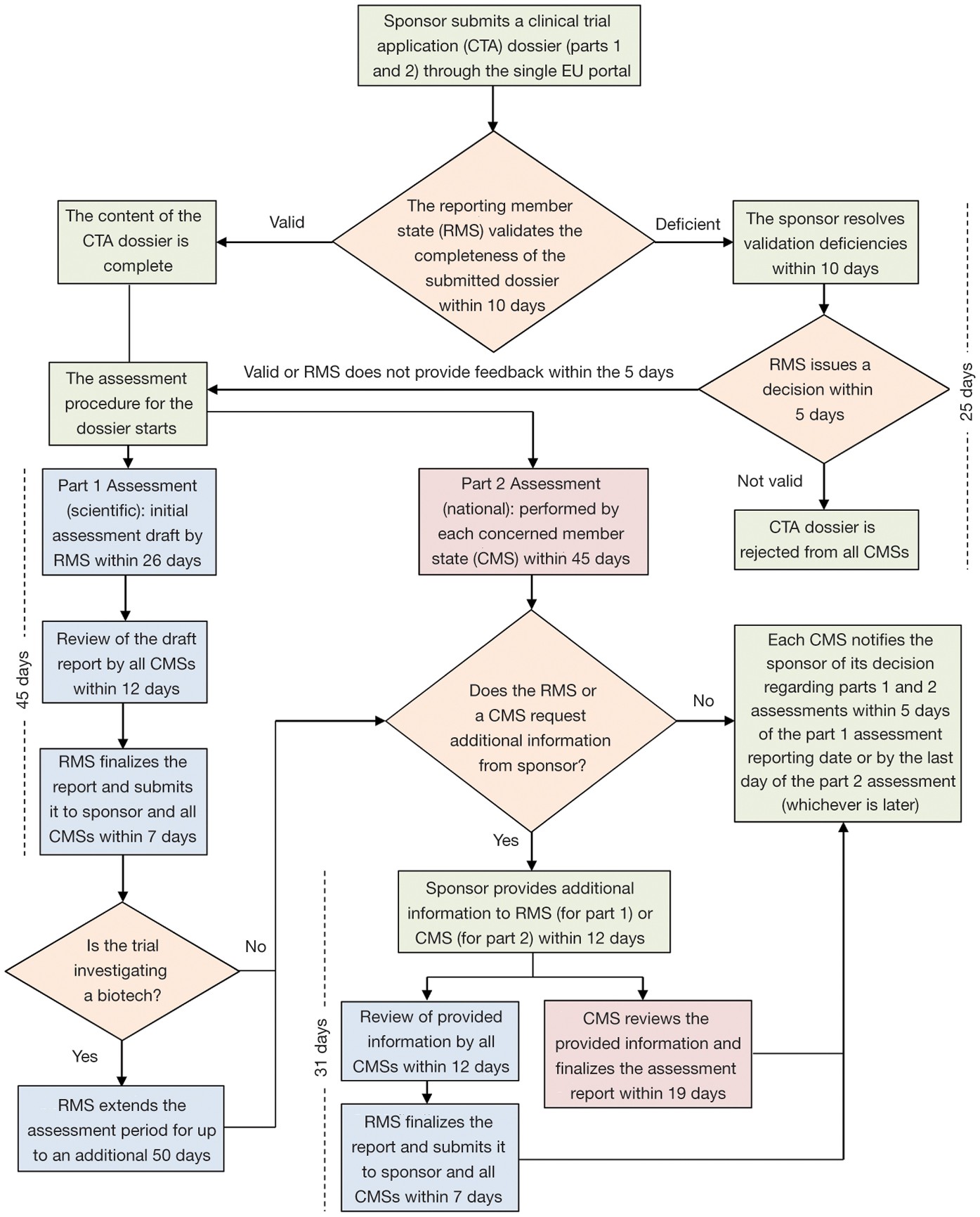
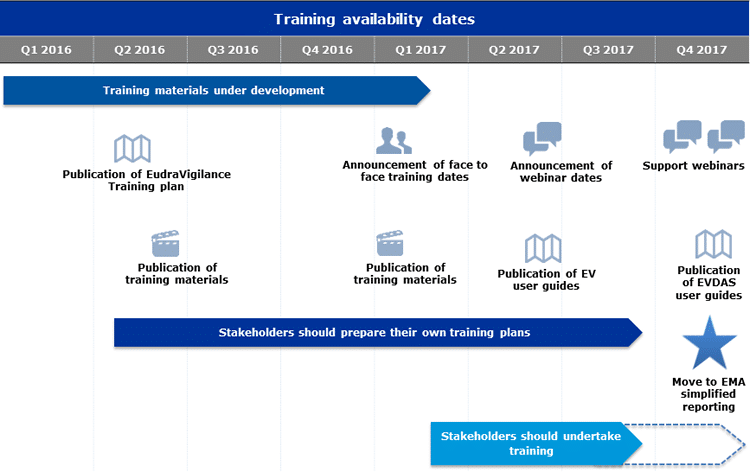
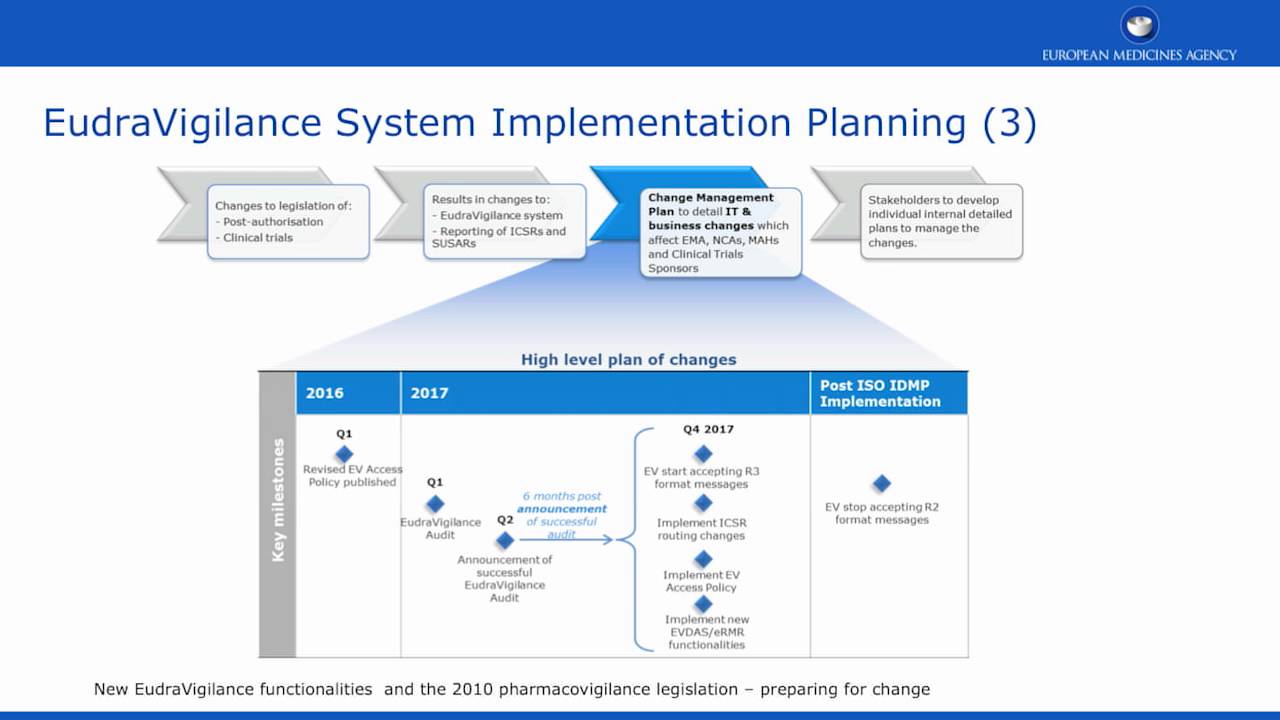
Q&A Document Available to Stakeholders Regarding Launch of the New EudraVigilance System - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety
Phone: +40-21 .31 7.11 .02 Fax: +40-21.316.34.97 Electronic Reporting of Suspected Unexpected Serious Adverse Reactions (SUSARs)
London, 01 February 2008 Doc. Ref. EMEA/553390/2007 NOTE FOR GUIDANCE EUDRAVIGILANCE HUMAN VERSION 7.1 PROCESSING OF SAFETY MESS