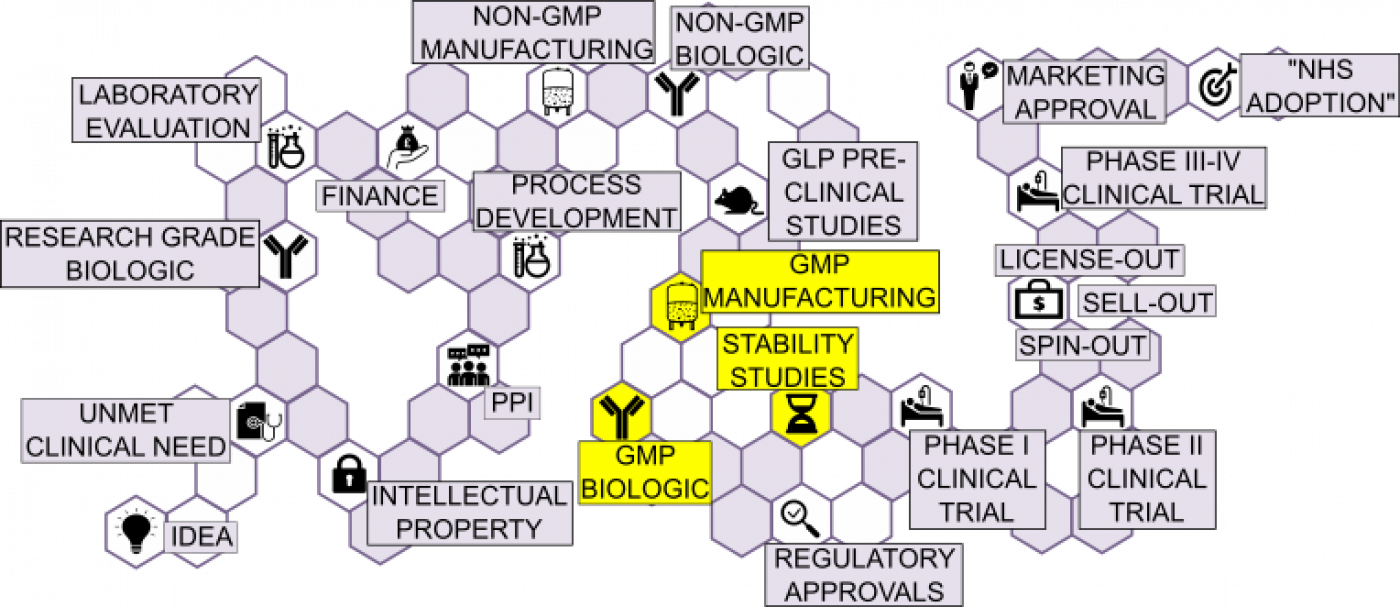
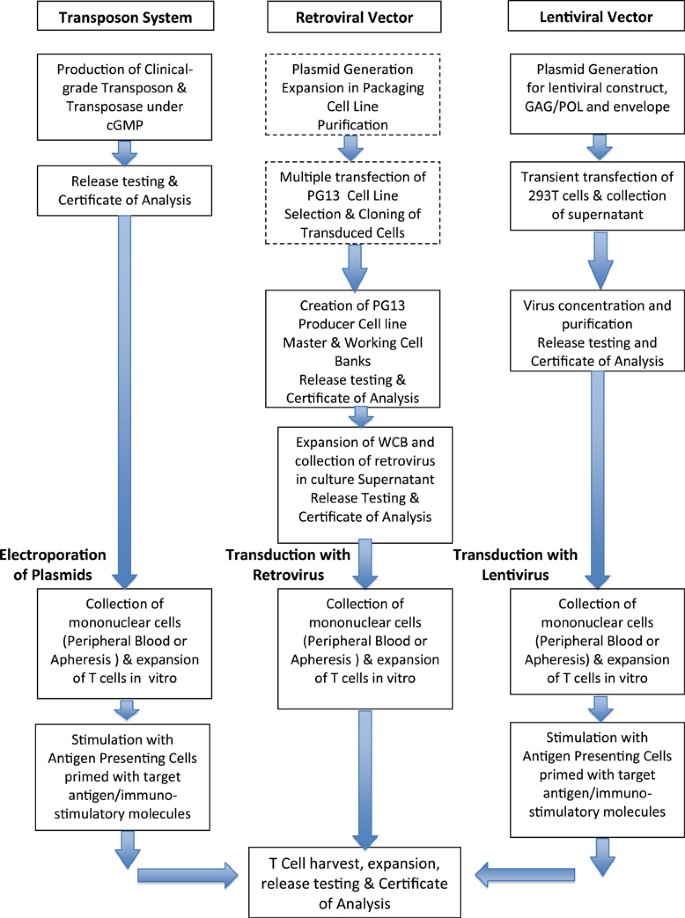
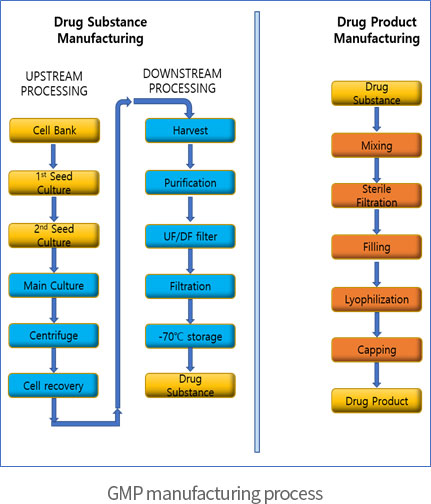
Challenges and advantages of cell therapy manufacturing under Good Manufacturing Practices within the hospital setting - ScienceDirect

Early Development GMPs for Drug-Product Manufacturing of Small Molecules: An Industry Perspective (Part III)

Vaccizone Selects Exothera for Process Development and GMP Manufacturing of its SARS-CoV-2 Vaccine | Technology Networks